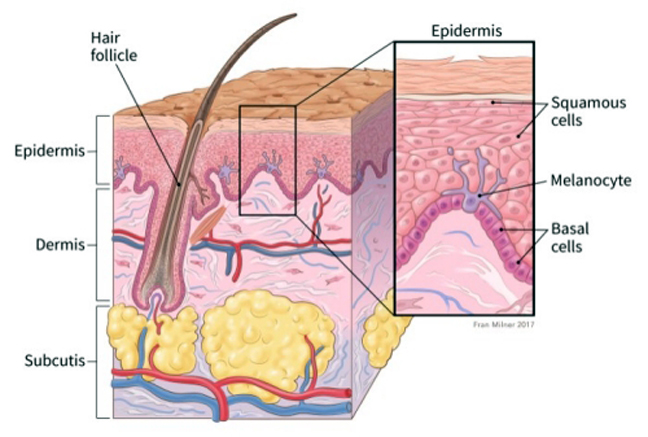
Regeneron Pharmaceuticals and Sanofi this morning jointly announced that the U.S. Food and Drug Administration will give priority review of the biologics license application for cemiplimab for the treatment of patients with metastatic cutaneous squamous cell carcinoma or patients with locally advanced CSCC who are not candidates for surgery.
Cemiplimab was invented by the Westchester-based science and technology company using its proprietary VelocImmune technology that yields “optimized fully-human antibodies.” It is being collaboratively developed by Regeneron and France-based Sanofi.
Earlier in the month, the European Medicines Agency accepted for review the marketing authorization application for cemiplimab.
Cemiplimab is “an investigational human monoclonal antibody targeting the checkpoint inhibitor PD-1 (programmed cell death protein-1) and was granted breakthrough therapy designation status by the FDA in September 2017,” according to the announcement. The target action date for the FDA decision is Oct. 28.
According to the American Cancer Society, 2 out of 10 skin cancers are squamous cell carcinomas.
These cancers commonly appear on sun-exposed areas of the body such as the face, ears, neck, lips and backs of the hands. They can also develop in scars or chronic skin sores elsewhere.
“Squamous cell cancers are more likely to grow into deeper layers of skin and spread to other parts of the body than basal cell cancers, although this is still uncommon,” according to the organization.




















