New York Medical College, a member of the Touro College and University System, is working to develop and bring to market what it describes as “a revolutionary new bandage using nanotechnology.”
The bandage is designed to deliver antibiotics, antiseptics and other materials directly to a wound while at the same time drawing out dead cells, bacteria and moisture to help keep the wound drier, cleaner and help it heal faster.
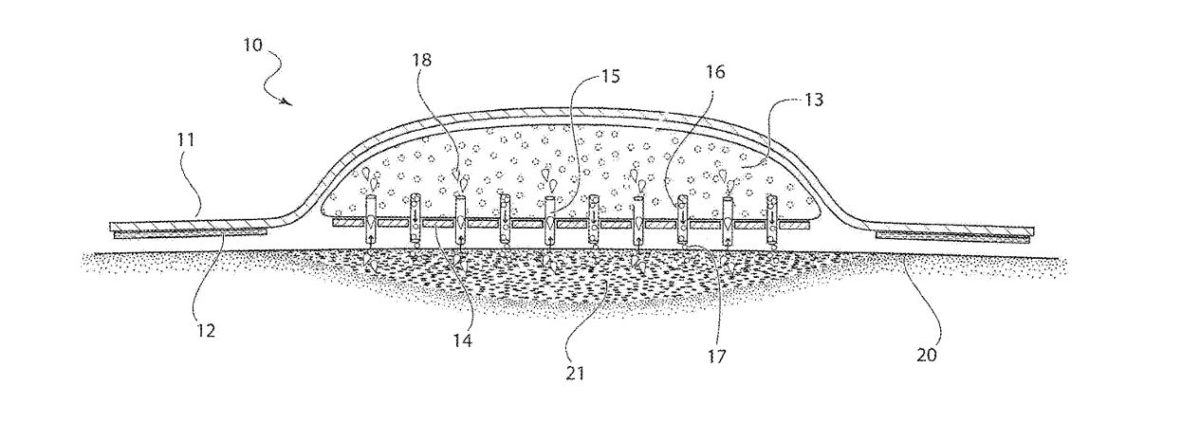
The U.S. Patent and Trademark Office today issued a patent for the bandage. The patent application was submitted by inventor Donald Spector. NYMC recently reached an agreement to acquire a number of Spector’s patents.
Dr. Alan Kadish, president of Valhalla-based NYMC and TCUS, announced the patent award. Spector had filed a patent application for the invention on Jan. 17, 2018.
“The new bandage protects a wound in the same way that any bandage does but also does two other things,” Kadish told the Business Journal. “One: it delivers drugs directly to the wound through microneedles that are embedded in the bandage. The second thing it does, it absorbs fluid from the wound to help drainage. The combination of those two things should dramatically help wound healing.”
Kadish said that the new bandage could be made in a variety of sizes and would be only slightly thicker than the self-adhesive bandages commonly used by consumers every day.
The new bandaging system has been given the trademarked name StatVac, “stat” referring to the commonly-used medical term “stat” for quickly or right now, and “vac” making reference to a vacuum, or reduction in pressure used to pull out material.
Kadish anticipated that the bandage would at first be aimed for use by physicians and in hospitals for more serious wounds but could some day find its way into anyone’s bathroom medicine cabinet to treat wounds using products contained in its microneedles such as antibiotic creams that the FDA has determined are safe and effective for use by consumers.
Making the product available for use by either professions or consumers will require approval by the Food and Drug Administration, although Kadish could not say at this point the particular path that would have to be followed through the approval process.
“Since there are devices that deliver antibiotics through external means, not the same way this bandage does, we believe it should be a relatively quick approval regardless of which path the FDA requires,” Kadish said.
“New York Medical College has had a several-year relationship with the primary inventor, Donald Spector,” Kadish said. “We’ve talked about this and other products and we’ve entered an agreement for New York Medical College to acquire a significant patent portfolio, which was developed by the inventor, in some cases with some collaboration, and New York Medical College now will do whatever is needed to further the development of these devices and hopefully license them.”
Kadish said the bandage that received the patent is still at the drawing board stage but there are some prototypes of earlier devices that are slightly different.
“We think that taking it from the development stage to a prototype stage should be relatively easy. Whether we do that ourselves or whether we do it in collaboration with a partner is something that we’re still trying to determine,” Kadish said.
“We have a good deal of research on campus. We’re not primarily a manufacturing facility so the likelihood is we’ll partner with someone to do the actual physical development. We don’t anticipate any major technological challenges to developing a prototype and developing a commercial product because it utilizes tried-and-true technology that shouldn’t be hard to utilize to make this smart bandage.”
Kadish noted that NYMC has various research programs and also operates the biotechnology incubator, Bioinc @ NYMC, which is about 4 years old now.
“It currently has 10 companies which work in a wide variety of different areas to try to develop products that improve patient health. We’ve had student involvement in some of those companies that are based in our incubator and we see this product and other similar products that will develop as being part of that effort, not focused on a specific area of strength that we have, but rather focused on areas that are cutting edge, that can develop new products that will make a difference in people’s lives and exposing our students to how those things develop,” Kadish said.
“The biotechnology incubator which is now full has been very successful at getting companies on their way to developing new products and exposing our students to that kind of exciting research.”
Kadish said he would hope to involve NYMC’s students in the process of product development and seeking FDA approval for the bandage.
When asked what it is that draws NYMC to wanting to create new medical products, Kadish said, “Our mission broadly is medical education, medical research and clinical care. This clearly involves the latter two. In clinical care, if it improves wound healing that’s a major advance. It certainly helps define our role in research; developing products to help healing. And, we hope to excite medical students by exposing them to the development of new technology and hope they collaborate in helping us do it, and then go on to being leaders in the development of medical technology in the future.”






















