Retia Medical becomes first BioInc@NYMC client to win FDA clearance
Retia Medical LLC has received federal clearance for a new cardiovascular monitor, the first company to obtain such approvals while a client of New York Medical College’s biotechnology incubator in Valhalla.
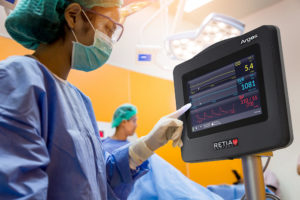
Retia Medical received U.S. Food and Drug Administration clearance for a cardiovascular monitoring system called the Argos Hemodynamic Monitor. It is now available for sale in the U.S.
Retia is among a group of medical and biotech startups that have offices at BioInc@NYMC, an incubator the college launched in 2014 on its campus at 7 Dana Road.
Hemodynamic monitoring helps measure cardiovascular output, the amount of blood pumped by the heart per minute. The Argos Hemodynamic Monitor is intended to provide care providers in an operating room or ICU with a more accurate and affordable monitor. According to its website, the 5-year cost to own the monitor is $19,200. Comparable monitors cost $84,000 and $90,000, according to Retia.
In a statement, CEO and co-founder Marc Zemel said the company “designed Retia”™s proprietary MBA algorithm to overcome the limitations of current (cardiovascular output) monitoring technologies in order to realize the potentially life-saving benefits that accurate and effective hemodynamic management can provide to patients.”
The company’s aim to is give clinicians up-to-the-minute data for patient care decisions. The company also hopes to offer clinicians greater access to cardiovascular output monitoring by reducing cost and inconvenience, factors it says limit use of the technology to the most high-risk patients.
New York Medical College, which is part of the metropolitan region”™s Touro College and University System, launched the BioInc@NYMC incubator with a mix of county, state and federal funds. The incubator received $1.25 million from the state in 2017 through its designation as a biotech Innovation Hot Spot.
BioInc@NYMC offers shared resources, lab space and professional services to entrepreneurs and startups in life sciences fields.
In 2017, the incubator booked a prominent anchor tenant when the Dutch technology conglomerate Royal Philips signed on for office space to house 13 researchers in its genomics program.
The incubator shares the building with the college”™s Clinical Skills and Disaster Medicine Training Center, a 21,000-square-foot facility that opened in 2014 as well.
Deborah Novick, director of BioInc@NYMC, said in a statement that the “recent advancement from Retia Medical is a reflection on the investment we have put into biological and medical innovation in the Hudson Valley.”