Norwalk’s BioWave pain-blocking tech expands from sports to veterans
If the “no pain, no gain” mantra has become something of a cliché, don”™t tell Brad Siff.
 The founder and president of BioWave, a pain-blocking technology increasingly gaining favor with athletes, physicians and military veterans, had already built a prototype of his device when he tore his anterior cruciate ligament and medial collateral ligament in his right knee during a skiing vacation.
The founder and president of BioWave, a pain-blocking technology increasingly gaining favor with athletes, physicians and military veterans, had already built a prototype of his device when he tore his anterior cruciate ligament and medial collateral ligament in his right knee during a skiing vacation.
“I was supposed to be in and out of the Hospital for Special Surgery in New York City in a day,” Siff said, speaking at his Norwalk office at 8 Knight St.. “But when I woke up I was in a lot of pain.”
Availing himself of a self-administered morphine pump only made matters worse. “My heart rate went way up, I was nauseous ”” I got really sick. I ended up spending three days in the hospital at huge additional expense,” he said.
The experience convinced him that BioWave could be the answer for a range of pain sufferers.
The company”™s first product was BioWavePENS, its acronym standing for percutaneous electrical nerve stimulation system. Essentially it works by delivering high-frequency therapeutic signals via electricity through skin into deep tissue, blocking pain signals to the brain.

Siff said that his technology is superior to TENS, or transcutaneous electrical nerve stimulation, procedures because the latter”™s low-frequency signals cannot pass through the skin.
“If you turn a TENS device up, you get a noxious, twitchy sensation that may distract from the pain but isn”™t really addressing it,” Siff said. “And when you turn it off, the pain comes right back.”
He compared BioWave”™s effect to that of novocaine, “except it”™s being delivered electrically instead of chemically.” A 30-minute treatment can provide up to 24 hours of relief, he said.
Siff received undergraduate and master’s degrees in engineering from Cornell University and an MBA from Cornell”™s Johnson Graduate School of Business. He said the school”™s affiliation with the Hospital for Special Surgery helped him get a meeting with the director of sports medicine for the New York Giants in 2005.
Lacking FDA approval for the BioWave at that point, Siff was unable to demonstrate its capabilities on players. But when an offensive line coach with arthritis in both knees tried it and was then able to do a squat with ease “jaws dropped,” Siff laughed.
Having received FDA clearance later that year, BioWave was soon being used regularly by the team. Siff said that about 600 treatments involving approximately 80 players were administered over the next 30 months.
Word of mouth soon spread and today BioWave is used by all 32 NFL teams, about 75 percent of both Major League Baseball and National Basketball Association squads, half of the National Hockey League teams and various Olympic and Major League Soccer players. All told, Siff said, “We”™re being used by over 90 professional teams at this point.”
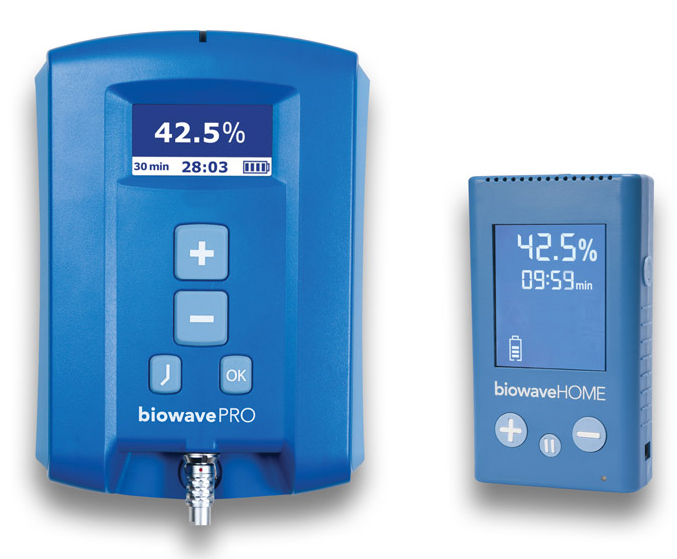
The sports market gave the company credibility, he said, and has helped it expand into the general population as well as the active military and veterans sectors. Twenty-nine VA hospitals now use the BiowavePRO, its device for medical professionals.
A third product, the BioWaveHOME, can be used by patients at home, usually with a doctor”™s prescription. The price for that device is $895, but Siff said the cost is usually picked up by Medicare and a number of insurers.
As BioWave is a privately held company, Siff declined to provide sales figures, but said that the company”™s 2017 revenues grew by “a little more than two times” over 2016, adding that he anticipates it to grow by at least two times more this year.
New products and applications are being tested for future rollout, Siff said. “I think there”™s a lot of opportunity for the company,” he said.