Bridgeport Hospital offers novel spray-on skin process for burn victims
We”™ve all heard of spray-on tans ”” but what about spray-on skin?
It may sound futuristic, but that future is already here at The Connecticut Burn Center at Bridgeport Hospital. The center’s medical director, Dr. Alisa Savetamal, said the process can save time and spare patients some of the pain associated with skin grafting.
“We”™re always looking for ways to improve care,” she said, “and this has a lot of promise.”
The process, which Bridgeport has been using for about four months, involves ReCell, the first product from Australian company Avita Medical. The ReCell device enables health care professionals to produce a suspension of spray-on skin cells using a small sample of the patient”™s own skin. The sample is processed in the operating room over 15 to 30 minutes, being mixed into a liquid that can be applied to the skin using a low-tech spray syringe.
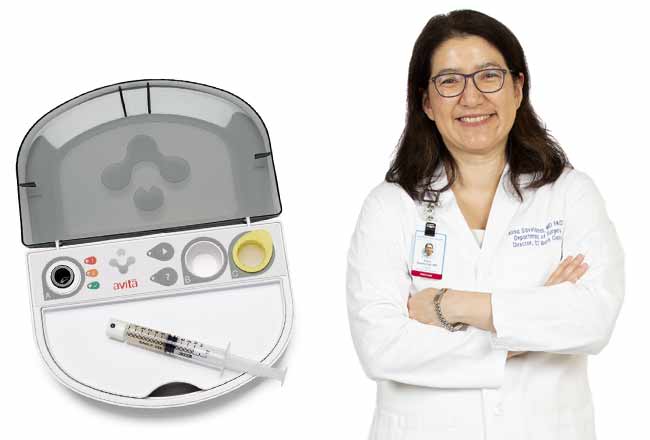
The cells in the solution then take hold and begin to grow, forming a fresh layer of normal skin.
“It”™s a new way of skin grafting but with much smaller donor sites,” Savetamal said, noting that the process can be especially beneficial for patients with large burns who may have limited healthy skin available for skin grafting.
“This is not eliminating skin grafting,” she said. “Depending on the size of the burn, ReCell can be used alone or in addition to grafting.”
For a standard skin graft, surgeons usually remove an area of healthy skin no smaller than three-quarters of the size of the wound, then stretch it over the damaged area. As the grafts use the top layers of skin, where most nerves are located, significant pain can be associated with the process.
“The benefit from ReCell for our burn patients is that they don’t have as large donor sites to heal in addition to their burns,” Savetamal said. “Fewer and smaller donor sites also mean less pain.”
The U.S. Food and Drug Administration approved ReCell last September ”” the first spray-on skin product to be granted such approval. Savetamal said that to date the burn center, the only one in the state, and one of only 65 in the nation, verified by the American Burn Association and American College of Surgeons for meeting national standards in the treatment of burns, has treated five patients, two of them with substantial burns, utilizing ReCell.
The burn center at Bridgeport Hospital treats about 230 patients a year, she said. According to the American Burn Association and the Centers for Disease Control and Prevention, each year nearly 500,000 fire or burn injuries were seen at emergency departments in the U.S. Avita estimates that inpatient treatment of burns in the U.S., the initial target for ReCell, is an approximately $200 million market.
Other companies are reportedly working on a variety of devices similar to ReCell, including Scottsdale, Arizona-based RenovaCare, whose SkinGun sprays a liquid suspension of a patient”™s stem cells onto wounds.
“We”™re very pleased with what we”™ve seen so far,” Savetamal said. “And we”™re very optimistic about using it going forward.”