Acorda seeks buyer as neurological drug sales fall
Rockland County pharmaceutical company Acorda Therapeutics Inc. has declared bankruptcy and is proposing a sale.
Acorda and five affiliates filed Chapter 11 reorganization petitions on April 2 in U.S. Bankruptcy Court, White Plains. They declared $109 million in assets and $266 million in liabilities.
“It is imperative that the company make a seamless transition into Chapter 11,” CFO Michael A. Gesser stated in a filing, “to preserve the patient supply of products, the reputation of the business, and the loyalty and goodwill of customers, suppliers, patients and employees.”
Acorda wants to sell by the end of the year. Merz Pharmaceuticals, of Frankfurt, German, has offered to pay $185 million for assets and to assume certain liabilities. Gesser said the company will also continue to solicit higher or better bids.
Acorda was founded in 1995 by medical doctor Ron Cohen. It is based in the Blue Hill Plaza in Pearl River.
It has developed medicines that treat Parkinson’s disease and multiple sclerosis.
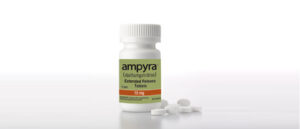
Ampyra, also known outside the U.S. as Fampyra, is an extended-release tablet that helps adults with multiple sclerosis walk better.
Inbrija is a dopamine replacement powder that treats motor fluctuations in adults with Parkinson’s disease.
But the company has struggled financially. Last year, Acorda recorded $118 million in net revenue, $253 million in net losses, and an accumulated deficit of $1.2 billion.
The company’s top seller, Ampyra, began losing market share after a court ruled against the company on a patent issue in 2018, allowing generic drug-makers to cut into sales.
In 2017, Ampyra netted $543 million in revenue. Last year it made $64 million.
In 2014, Acorda acquired the rights to Inbrija for $525 million while the drug was undergoing clinical trials. The U.S. Food and Drug Administration approved the product in 2018 and it was put on the market in 2019.
Acorda expected Inbrija to make $300 to $500 a year in net revenues, according to Gesser, but annual sales never exceeded $38.4 million.
He attributed the results to Covid-19 pandemic travel disruptions that made it more difficult for Parkinson’s patients to see doctors and to shelter-in-place orders that decreased patients’ mobility needs.
In 2016, the company paid $363 million for the rights to Tozadenant, a promising Parkinson’s drug that was in clinical trials. But in 2017, “after serious adverse events occurred in patients during phase 3 trials,” Gesser said, development was discontinued. The medicine never made money.
Acorda reduced its workforce and expenses four times from 2017 to 2021, according to Gesser. But it failed to generate sufficient cash flow or capital, and the company was unable to invest in other products.
The company also owes about $207 million on a debt that will mature in December.
Selling assets would be the best way to maximize value for company stakeholders, Gesser said, while ensuring that “products would continue to be provided on an uninterrupted basis to patients who benefit from these much-needed medications.”
Acorda is publicly traded on the Nasdaq exchange. It’s high for the past year was $17.60 per share on Aug. 7, 2023. It closed at $13.47 the day before it filed for bankruptcy protection. The day after, it closed at $1.99 per share.